Abstract
Background: Acute myeloid leukemia (AML) is a heterogeneous hematologic malignancy with high mortality and poor outcome. Highly effective therapy options are always needed for AML. Selinexor (KPT-330) is an oral bioactive selective CRM1 (XPO1) inhibitor, and the clinical trial showed the combination of KPT-330 over standard induction therapy benefits much for AML patients. Azacitidine (AZA), a DNA hypomethylation agent, can induce cell apoptosis and exhibit potential therapeutic efficacy against AML. However, it has not yet determined the anti-tumor effect of KPT-330 with AZA in AML. Here we examined the anti-leukemia effect and potential mechanism of KPT-330 combined with AZA in AML cells to provide evidence for further clinical trials.
Methods: A total of 64 AML patients’ BM samples and 64 normal control from healthy volunteers were obtained from Zhongda Hospital Southeast University, in which the 2 patients’ samples were further treated with indicated drugs in vitro. Cell Counting Kit-8 (CCK-8) was used for cell viability assay, Annexin-V/PI staining following flow cytometry analysis for apoptosis, and qPCR and Western Blot for gene expression in AML cell lines and patient samples. CalcuSyn analysis was performed for a synergistic effect. RNA-seq was performed in U937 cells treated with 0.7uM AZA for 48 hrs, and the significantly differentially expressed genes (DEGs) were identified and gene set enrichment analysis (GSEA) was performed.
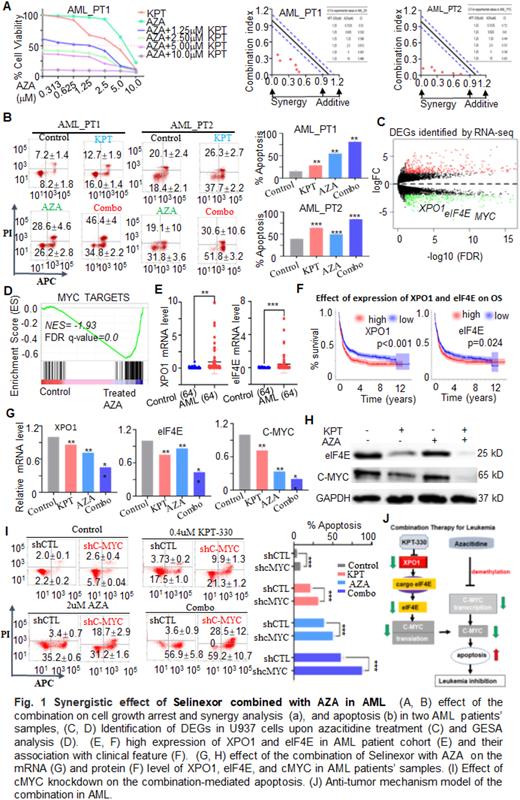
Results: Cell proliferation arrest and apoptosis were significantly observed in U937 and MV4-11 cells treated with the combination of KPT-330 with AZA compared to single drug control (data not shown). Then, we further explored the synergistic effect of the combination in patients’ samples. Results showed that cell growth arrest and apoptosis significantly increased in primary samples from two de novo AML patients compared to the single drug controls, and CalcuSyn analysis showed a synergistic effect of the combination on cell growth arrest (Fig. 1A &1B). To understand the mechanisms underlying the synergy, RNA-seq analysis was performed in U937 cells treated by 0.7uM AZA and 4655 DEGs were identified. XPO1, eIF4E, and MYC are in the top DEGs upon AZA treatment, and GSEA analysis indicated MYC_TARGETS and apoptosis pathway are enriched in DEGs (Fig. 1C &1D ). Moreover, the mRNA level of XPO1 and eIF4E was increased in our AML patient cohort compared with healthy controls (Fig. 1E), and the high expression of XPO1 or elF4E are associated with poor outcome (Fig. 1F). XPO1 is the key component of nuclear-exporting machinery; elF4E, the cargo of XPO1 is translation initiation factor and plays a critical role in translational machinery, which regulates the C-MYC translation. It is also reported that AZA regulates the transcription of C-MYC by affecting methylation. We observed a combination of KPT-330 with AZA significantly down-regulated the mRNA and protein level of oncogene eIF4E and C-MYC in two of the patients’ samples compared to single drug controls (Fig. 1G &1H ). Also, C-MYC knock-down was not only significantly increased the apoptosis in U937 cells compared to scramble shRNA (shCTL) control, but also significantly enhanced the combination-mediated apoptosis compared to that of single drug control in U937 cells (Fig. 1I). these data indicated that XPO1/eIF4E/C-MYC signaling plays critical roles in the combination-mediated anti-leukemia, and the mechanistic model is summarized in Fig.1J.
Conclusions: Our data for the first time showed the synergistic effect of a novel combination of KPT-330 with AZA on cell growth arrest and apoptosis in AML patients and also identified the underlying mechanism by targeting XPO1/eIF4E/C-MYC signaling. Our results also highlighted the feasibility of the clinical trial for the combination in the therapy of AML patients.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal